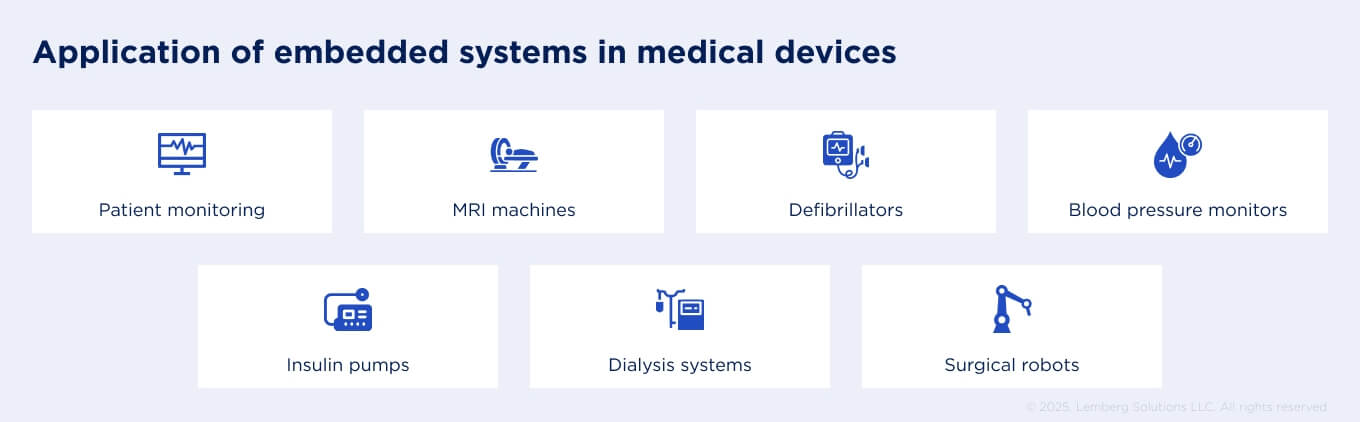
From wearable sensors and patient monitors to insulin pumps and surgical robots, embedded software lies at the core of today’s healthcare technology trends. It ensures patient safety, reliable performance in critical environments, and long product lifecycles of 10–15 years.
Still, developing an embedded medical device is never a straightforward process. It is shaped by regulatory requirements, technical complexity, and hidden costs. However, with the right plan, you can prevent costly delays, achieve certification, and deliver safe, innovative products to market faster. Success requires a structured approach:
- Validate the need: assess market demand and contractual obligations early.
- Maintain compliance: map the regulatory standards and certifications your device must meet.
- Design smart: choose an architecture and risk strategy that balances innovation with reliability.
- Partner wisely: collaborate with the right software engineering experts.
Equally important is knowing what to avoid: underestimating budgets, ignoring scalability, neglecting quality management, or skipping security-by-design.
This article provides a practical, step-by-step blueprint to help startups and R&D teams navigate the complexity of embedded medical device development, turning ideas into market-ready solutions.
How to start embedded software development for a medical device
Medical device development extends beyond technical requirements. Certainly, many issues are associated with embedded design, which comprises processors, storage units, sensors, communication protocols, and user interfaces. However, legal and contractual requirements set the pace from the very start of the development process, forcing companies to navigate multiple requirements throughout the software lifecycle, risk management, safety, and performance.
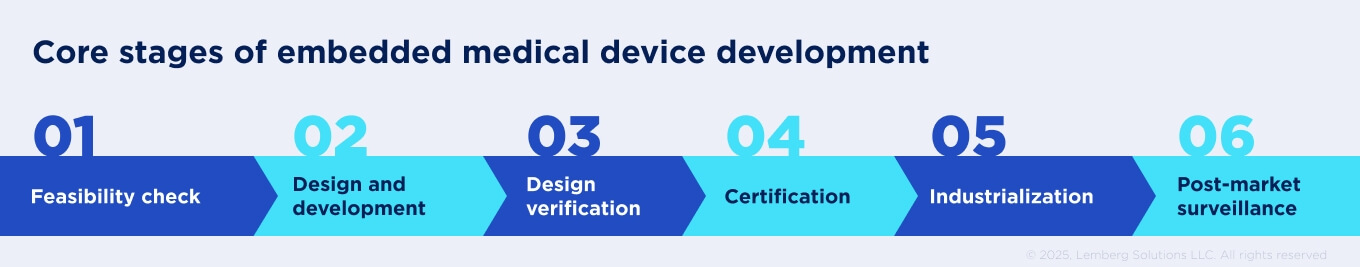
Core stages of embedded medical device development include:
Feasibility check
If regulatory requirements are considered from day one, the device can progress smoothly through development, verification, clinical evaluation, and post-market surveillance.
At this stage, companies analyze the clinical problem, the market opportunity, and the regulatory pathway. This includes evaluating applicable regulations and standards and estimating the economic viability of the project. At this stage, companies analyze the clinical problem, estimate the economic viability of the project, and evaluate applicable regulations and standards.
Design and development
This stage involves defining both functional and non-functional requirements, encompassing safety, performance, cybersecurity, and usability expectations. At this point, embedded teams are expected to create a detailed project plan and develop early prototypes of the device.
Design verification
Verification ensures that the implemented design meets the defined requirements and complies with relevant standards and regulations. For embedded software, this may include testing, real-time performance validation, and documentation of traceability. The goal is to demonstrate that the software and hardware components operate safely and reliably under intended use conditions.
Certification and qualification
Before entering the market, the device must show compliance. This may involve clinical evaluations or trials, cybersecurity assessments, electrical safety and EMC testing, and software validation to meet FDA, EU MDR, etc.
Industrialization and post-market surveillance
At this stage, the focus shifts to scaling production while maintaining quality and regulatory compliance. Once commercialized, devices are continuously monitored for safety, performance, and user satisfaction. For embedded systems, this includes releasing software updates, security patches, and feature enhancements through validated processes such as OTA (over-the-air) updates. Post-market activities must be documented in accordance with national requirements (FDA post-market guidance and EU MDR vigilance requirements).
Compliance roadmap: key standards and regulations
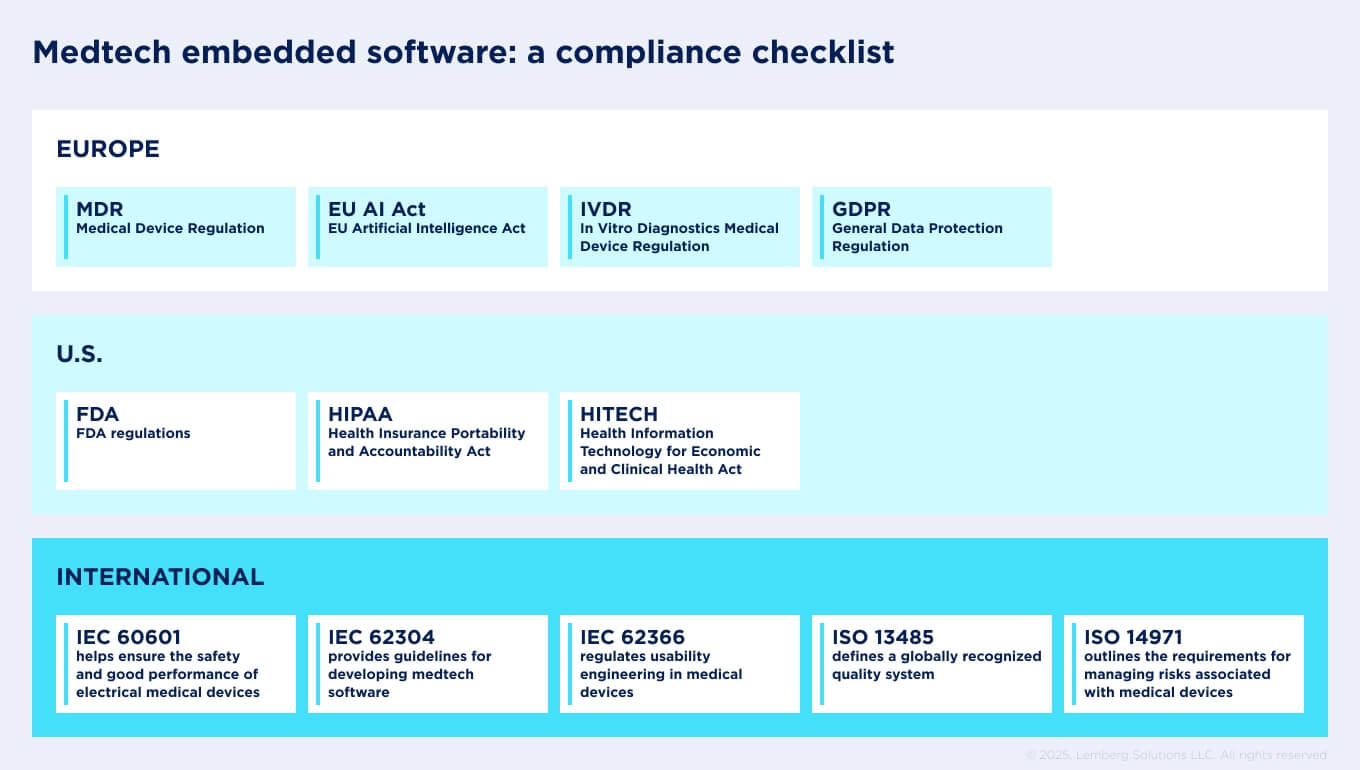
Any business seeking to introduce a device on the market, whether in Europe or the U.S., must comply with strict regulatory requirements.
European regulations on developing software for medical devices
In Europe, the primary regulations governing medical devices are the Medical Device Regulation (MDR) and the In Vitro Diagnostics Medical Device Regulation (IVDR). GDPR (General Data Protection Regulation) and EU Artificial Intelligence Act also have a great impact.
MDR
The MDR sets the rules for developing, testing, approving, and monitoring medical devices across EU countries. Its goal is to make sure that devices placed on the European market are safe and traceable throughout their entire lifecycle. MDR applies to different products, including bandages and syringes, as well as to complex technologies such as pacemakers and dialysis systems.
IVDR
The IVDR governs in vitro diagnostic (IVD) medical devices that test samples, such as blood. Unlike MDR, which covers therapeutic and monitoring technologies, IVDR applies to pregnancy tests, glucose strips, and advanced laboratory analyzers.
GDPR
Medical technologies should be developed with privacy in mind. For medtech startups in the EU, GDPR sets the ground rules for building secure, privacy-by-design medical devices. Developers must follow the regulations by embedding encryption, consent management, and secure data flows into the software architecture. This adds complexity and cost but also builds trust with patients and regulators.
EU AI Act
Developers now frequently incorporate AI into medical devices. The EU AI Act is shaping this process by classifying most medical AI as “high-risk,” which raises the bar for compliance. For software teams, that means building transparency, explainability, and human oversight into algorithms from the start. It also requires strict attention to data quality, bias reduction, and auditability, making development more complex but ultimately leading to safer, more trustworthy AI-driven devices.
U.S. regulations on developing software for medical devices
Healthcare compliance for medical devices in the USA is based on HIPAA (Health Insurance Portability and Accountability Act), as well as the regulatory framework established by the U.S. Food and Drug Administration (FDA).
FDA
The FDA is the federal agency responsible for regulating the development, approval, and monitoring of medical devices in the United States. Its regulatory framework is based on a risk classification system (Class I, II, III), with higher-risk devices requiring more rigorous oversight. The FDA also enforces quality standards under 21 CFR Part 820 (Quality System Regulation), which requires manufacturers to establish processes for design controls, risk management, software validation, and corrective actions. This system ensures that medical devices sold in the U.S. are safe, effective, and manufactured under strict quality oversight.
HIPAA & HITECH
While not device-specific, HIPAA & HITECH directly affect how connected medical devices are designed and brought to market. Any device that collects, stores, or transmits protected health information (PHI) must comply with HIPAA’s Privacy, Security, and Breach Notification Rules. For developers, this means that cybersecurity safeguards, encryption, access controls, and audit trails must be built into the device from the earliest design stages, ensuring regulatory compliance and protecting patient trust.
International standards on developing software for medical devices
Along with regulations, there is a set of internationally recognized standards that guide the development of embedded software for medical devices, regardless of country or region. We have prepared a checklist that helps startups and R&D teams align their development process with these requirements from the very beginning.
IEC 60601
IEC 60601 (General Requirements for Basic Safety and Essential Performance) is a series of technical standards designed to ensure the safety and good performance of electrical medical devices. It includes the general standard (IEC 60601-1), collateral standards (IEC 60601-1-x) for specific aspects applicable to certain devices, and particular standards (IEC 60601-2-x) addressing requirements for specific medical products.
IEC 62304
The IEC 62304 (Medical Device Software — Software Lifecycle Process) provides guidelines for developing software for medical devices. For that, it defines a lifecycle with activities and tasks necessary for the safe design, development, and maintenance of medical device software. IEC 62304 is applied to all medical devices that incorporate software as a core or relevant part.
IEC 62366
IEC 62366 (Medical Devices Engineering to Medical Devices) regulates usability engineering in medical devices, helping manufacturers design user-friendly products and reduce errors associated with complex or poorly designed interfaces. The standard is divided into two parts. IEC 62366-1 (2015) defines the required processes for analysing, designing, verifying, and validating usability. IEC 62366-2 (2016) provides guidance on how to apply Part 1.
ISO 14971
ISO 14971 (Application of Risk Management to Medical Devices) outlines the requirements for managing risks associated with medical devices and provides a structured framework for identifying, assessing, and controlling safety issues. The standard outlines processes for establishing a comprehensive risk management system, which involves evaluating risks to patients, operators, and other users, and implementing appropriate control measures.
ISO 13485
ISO 13485 (Quality Management) defines a globally recognized quality system to ensure that an organization has the required procedures in place to design, manufacture, and maintain a reliable and high-quality medical device. The standard implementation involves establishing procedures for planning, requirements definition, design control, change management, verification and validation, documentation, and transfer.
Architecture & design choices for embedded medical devices
When developing embedded medical devices, architecture and design choices directly influence safety, usability, and compliance. And the right option depends heavily on the setting and use case.
RTOS vs. bare-metal
In essence, while bare metal systems promise efficiency and direct control, RTOS solutions provide a structured environment that simplifies the development of complex applications and ensures real-time constraints are met.
You can design your medical device either with or without an operating system. Generally, embedded software designers offer real-time operating systems (RTOS) or bare-metal systems that differ in their architecture, capabilities, and implementation.
In a bare-metal system, applications run directly on the hardware, without any intermediate software layers. In practice, these systems are used with non-complex, single-function devices that require ultra-low power embedded design and minimal latency (e.g., glucose meters, basic sensor nodes).
An RTOS, by contrast, provides an environment that lets several tasks run in parallel. It fits multitasking systems that need scheduling, connectivity stacks, and updates (e.g., infusion pumps, patient monitors).
Connectivity
Connectivity is another critical architecture choice for embedded medical devices. There are several options for this:
- Bluetooth Low Energy (BLE) is well-suited for wearables such as glucose monitors or insulin pumps that rely on periodic data transmission. BLE connects through a nearby smartphone, making it a good fit for patient-friendly devices. Yet, it can create accessibility challenges for seniors.
- Wi-Fi is a better fit for devices that must stream continuous high-volume data directly to clinicians. These include wearable ECG monitors, hospital bedside monitors, and home telehealth kits. Its ability to integrate with hospital EHR systems in real time makes it essential for inpatient care, although its power consumption and reliance on stable local infrastructure may pose some limitations.
- Cellular IoT enables devices to connect directly to the cloud without the need for a phone or router. Such independence is highly effective for glucose monitors (CGMs) or connected blood pressure cuffs that support chronic disease management and need continuous communication.
It’s worth mentioning that all wireless technologies in Europe are regulated by the EU RED (Radio Equipment Directive). Similar regulation for the American market is the U.S. FCC.
- Finally, wired connections such as Ethernet, USB, RS-485, or CAN bus remain vital in stationary diagnostic equipment (ECG machines, dialysis systems), operating room technologies (surgical robots, anesthesia devices), or medical imaging systems (CT, MRI, ultrasound). In these contexts, reliability, high bandwidth, and electromagnetic compatibility far outweigh the convenience of wireless mobility.
Secure boots vs. OTA updates
Ensuring the security of embedded medical devices has become non-negotiable, especially as cyber threats become increasingly sophisticated. Among the most important mechanisms in this domain are secure boot and OTA updates.
Secure boots validate the device’s firmware and software components using cryptographic signatures. When properly implemented, they prevent unauthorized or malicious software from loading, making it ideal for devices such as infusion pumps, implantable devices, and patient monitors.
OTA updates let manufacturers deploy patches, bug fixes, and even feature upgrades after devices are already in use. This is critical because medical devices are typically designed to have a long-term lifespan. The FDA’s premarket cybersecurity guidance specifically expects devices to have secure update mechanisms.

Best practices for medical device software development
Developing embedded software for medical devices requires strict compliance with regulatory and contractual requirements. To achieve it, teams should follow these practices:
- Documentation and traceability. Maintain complete design history files, risk assessments, and requirement traceability to meet regulatory requirements and support efficient audits.
- Risk-based design and validation. Apply ISO 14971 principles to identify, evaluate, and mitigate risks at every stage of medical device development, ensuring that safety considerations drive design decisions.
- Automated testing and CI/CD for embedded systems. Utilize unit and integration tests, and continuous integration pipelines to identify defects early in development, enhance reliability, and expedite release cycles.
- Cross-functional collaboration. Foster close cooperation between hardware engineers, software developers, QA engineers, and regulatory experts to align technical performance with compliance and patient safety requirements.
- Secure development practices. Embed cybersecurity measures such as threat modeling, encryption, trusted software libraries, and secure boot into the development lifecycle, in line with officially accepted cybersecurity guidelines.
- Post-market readiness. Plan from the start for software maintenance, OTA updates, and incident response processes to meet post-market surveillance obligations under MDR, IVDR, or FDA requirements.
Common mistakes startups make in embedded software development for medical devices
The average failure rate of healthcare startups is currently 44%, with 10% of newly launched startups unlikely to survive in the industry within their first year. Complex and constantly changing regulatory requirements, stringent quality and safety standards, high development and manufacturing costs, and lengthy approval processes are the primary reasons for this. Some of the common mistakes startups make when developing their devices include:
- Compliance as an afterthought
- Failure to plan the budget properly
- Neglecting quality management
- Ignoring scalability issues
- Lack of cybersecurity-by-design
Let’s see them in more detail:
Compliance as an afterthought
Many teams jump straight into building device prototypes, leaving regulatory questions for later. In medical device development, however, failing to classify your product properly or neglecting key regulations at an early stage can disrupt the whole project. In heavily regulated industries like healthcare, regulations dictate how you organise your work. They determine how you run the entire development process, what documentation you need, how you manage risks, and how you validate results.
Failure to plan the budget properly
The reality of hidden costs suggests it’s smart to plan for at least a 30% overestimate.
Many medtech startups underestimate the true costs of developing a compliant medical device. Expenses for documentation, testing, regulatory submissions, and quality management often exceed initial projections significantly. Without a realistic budget, companies risk running out of funds, delaying approvals, or cutting corners that compromise safety and compliance.
Additionally, many companies budget solely for prototype design and overlook post-market requirements, such as surveillance, quality system upkeep, and manufacturing validation.
Neglecting quality management
Neglecting quality management is a common pitfall for medtech startups. In the rush to build and launch their products, teams sometimes treat quality processes as paperwork rather than a foundation for product safety and compliance. Without a proper quality management system (QMS) aligned with standards like ISO 13485, startups risk failed audits, costly redesigns, and delays in regulatory approval.
Ignoring scalability issues
Launching a medical device is just the beginning. Without scalability in mind, problems multiply as soon as your devices are in use. Startups often overlook key questions: how will bugs be fixed once devices are in the field? How will new features be added? What happens when regulatory requirements or language support change as you expand into new markets? Every update must be validated to prove the device still works as intended, which makes service planning for upgrades, bug fixes, and enhancements essential.
Lack of cybersecurity-by-design
Startups sometimes treat security as an afterthought, adding it late in the development process rather than building it into the product from the beginning. For medical devices, this creates serious risks: weak encryption, unprotected communication channels, and poorly managed access can expose sensitive patient data or allow unauthorized device control. Regulations increasingly expect manufacturers to demonstrate security throughout the product lifecycle, not just at launch. Without cybersecurity-by-design, companies face a higher risk of breaches, compliance failures, and a loss of trust from both regulators and end-users.

Balancing speed & compliance: how to reduce time-to-market
Embedded software development for medical devices is part of playing a long-term game. Many startups are looking for ways to reduce time-to-market while trying to adhere to medical device regulations.
The good news is that it’s possible with the right strategy and early alignment to standards.
Make regulatory compliance your starting point. Reducing the time-to-market for medical devices begins with incorporating regulatory compliance into the development process from the outset.
Another important strategy is to use pre-certified components and platforms. Selecting microcontrollers, wireless modules, or operating systems that already meet recognized standards can reduce the verification and validation steps. For example, many manufacturers rely on pre-certified Bluetooth modules or RTOS with safety documentation to cut down testing time.
Ultimately, companies can expedite development by collaborating with experienced engineering teams that comprehend both the technical and regulatory landscapes. Expertise in areas like embedded firmware, usability engineering, and compliance can prevent costly redesigns.
Choosing the right partner for embedded software development for medical devices
Bringing a medical device to life takes more than just coding skills. You need a team that knows embedded engineering inside out, understands regulatory standards, and can back it all up with strong QA and IoT security expertise. When those competencies come together, the development process runs a lot faster.
Outsource complex tasks like firmware development, verification, and validation testing. Delegating these responsibilities to an experienced partner reduces risk, accelerates compliance, and frees your internal team to focus on strategy and innovation.
That’s where Lemberg Solutions comes in. With hands-on experience in embedded medical projects and a track record of guiding clients through regulatory complexity, they help startups and medtech companies shorten time-to-market without compromising compliance.