Looking to launch your healthcare product on the US market but still hesitate, which standards and regulations to keep in mind?
In this article, we collected industry regulatory compliance influencing healthcare software development and outlined strategies to comply with them. Keep reading to navigate healthcare standards and regulations while building and launching your product.
You can explore this topic further by watching our video on our Youtube channel.
Healthcare standards and regulations for medical devices in the USA
Complying with these standards and regulations ensures you launch digital healthcare products at a lower time-to-market, providing future patients and healthcare units with quality and safe solutions.
Regulations for medical devices in the USA
FDA regulations
The Food and Drug Administration (FDA) is an American federal agency responsible for ensuring the safety, efficiency, and security of medical devices that enter the market. By medical devices, we mean all software and hardware solutions that are used for medical purposes and can access patient health information.
- How does it affect software and device development in healthcare?
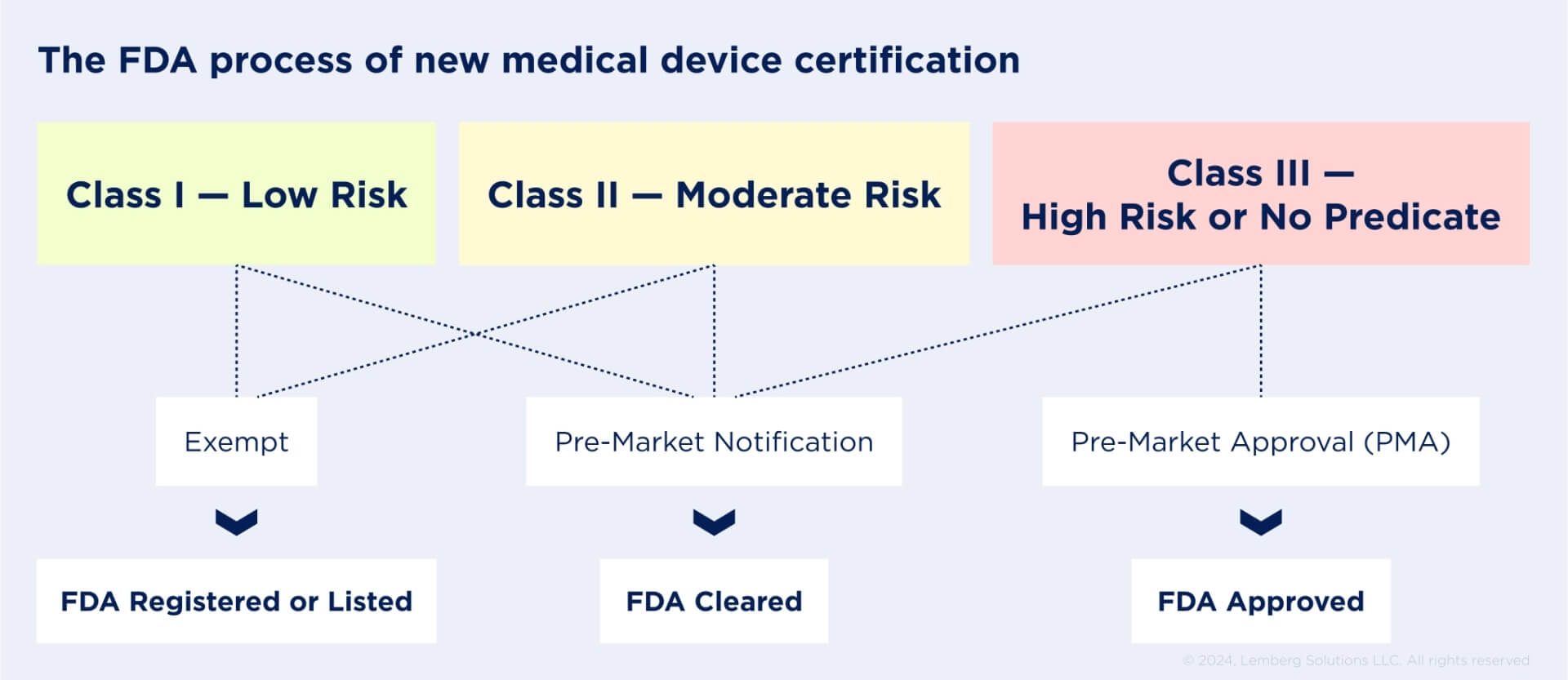
FDA provides requirements for the manufacturing, importing, marketing, and selling of medical devices in the United States. The FDA Center for Devices and Radiological Health (CDRH) regulates firms that produce, repackage, relabel, and/or import medical devices sold in the USA.
The regulator defines the safety and effectiveness of your medical device, determines whether it complies with all industry standards, and classifies your solution by medical specialty and regulatory classes based on risks patients can be exposed to.
21st Century Cures Act
The 21st Century Cures Act (Cures Act) is another newly launched law aimimg to streamline the medical device development process and improve medical system interoperability.
It speeds up the medical device regulatory process, including the proper classification and correspondence to industry standards. In addition, the law also integrates interoperability standards that define policies for developing and implementing APIs in medical systems.
Also, the Act incorporates new medical product development and certifying programs like the Breakthrough Devices that establish new medical solutions reviewing processes, driving innovations in the healthcare industry.
- What influence does the Cures Act have on medical software development?
As the incorporation of the Cure Act enables the interoperability of healthcare software and devices, patient data security becomes more vulnerable, requiring the highest protection.
To reach high-security measures, your medical solution must comply with all medical devices compliance laws like HIPAA and HITRUST, which regulate the usage of patient data within health IT systems.
It should also be integrated with secure communication protocols to ensure a smooth and protected data exchange between different systems and parties, including hospitals, laboratories, pharmacies, and insurance companies.
Quality and safety standards for medical device development

ISO 13485
ISO 13485 is an international standard for quality management systems of medical devices that cover requirements for developing, implementing, and maintaining solutions. Following these requirements while designing and developing medical devices (including SaMD and SiMD) ensures the building of safe and efficient solutions that are compliant with industry standards globally and meet user needs and expectations.
Once a medical device is delivered, it must go through the Medical Device Single Audit Program (MDSAP) audit process. This program is recognized by the FDA as the one that satisfies the requirements of multiple regulatory jurisdictions in collaboration between regulatory authorities in the USA, Canada, Japan, Brazil, and Australia.
- How does ISO 13485 influence the medical device development lifecycle?
If you want to launch your medical device with a reduced time-to-market, adherence to ISO 13485 compliance is a must from the outset of the product development lifecycle.
This compliance provides a ready-made framework for developing and launching a medical solution. Moreover, complying with this regulation ensures your product will pass through other medical device compliance, like those established by the FDA.
As a result, you deliver safe and industry-compliant medical devices and establish a reputation as a credible and trusted business in the healthcare sector.
IEC 62304
Among the standards manufacturers can use to demonstrate compliance with essential principles of safety and effectiveness, IEC 62304 is particularly recommended for medical device software development.
IEC 62304 is a safety standard that defines the medical software development and maintenance lifecycle, including risk management. This compliance divides all medical devices into three classes, depending on their medical intention and safety risk.

Following IEC 62304 from the beginning of medical device development is a mandatory industry requirement, as it mitigates patient risks and ensures product quality when entering the market.
- How does IEC 62304 affect healthcare software projects?
After you’ve developed the medical device, IEC 62304 requirements cannot be neglected if you want to launch the product.
First, the FDA will conduct a regulatory assessment of your product. Once you get approval, your product can be launched and used as intended.
In case the FDA reveals a mismatch with IEC 62304 development frameworks, your launch request will be denied and postponed until all IEC 62304 requirements are met.
So, when looking for a provider to develop your solution, ensure they are proficient with this compliance and adhere to its requirements in their development lifecycle.
Data security laws and rules to consider for medical solutions

HIPAA
HIPAA is a US federal law regulated by the Department of Health and Human Services (HHS) that provides industry standards for using protected health information (PHI). It spans all types of data related to each particular patient, including medical records, prescriptions, lab test results, insurance, and billing information.
- What is the importance of HIPAA compliance in healthcare development?
HIPPA privacy rules create standards to protect individual's health information, following the CIA triad — confidentiality, integrity, and availability. So, why is HIPAA compliance crucial to consider while developing your medical solution?
- HIPAA-compliant solutions can enter the market and be used on purpose much quicker.
- Neglecting the HIPAA-compliant approach in healthcare software development leads to getting hefty penalties and lawsuits for your business.
- Mismatching with HIPAA requirements might cause a health data security breach that will completely disrupt your customers' trust and ruin your brand’s image, which may even result in a business shutdown.
You can also learn about DiGa, European healthcare compliance considered analogous to the USA's HIPAA. Follow the link to discover the requirements this directory provides and why medical devices must comply with them to get launched to the market.
HITECH Act
The HITECH Act is one of the American Recovery and Reinvestment Act regulations that enable healthcare units to digitize medical records, applying HIPAA regulatory compliance, and assisting facilities in this transition.
It aims to enhance the US healthcare system by improving the quality and safety of medical services, privacy, security, and easier access to health data, as well as developing patient self-care.
- How does it affect healthcare product development?
As the HITECH Act applies to EHR and financial incentives for healthcare organizations to transit all medical records into electronic format, it sets specific requirements for medical software that stores and uses electronic health records:
- Compatibility with HIPAA regulatory standards to ensure privacy and security for PHI and oversee how medical records will be used and accessed within software systems.
- To get an EHR system certified, it is crucial to follow the rules for keeping, using, and exchanging medical records between medical devices and systems.
- Healthcare software development's primary focus must be protecting patient data from breaches and improving the quality of care.
HITRUST
HITRUST Framework focuses on patient health data security used within health IT systems by integrating requirements to data management and safeguarding, as well as risk assessment and management.
HITRUST is commonly compared to ISO 27001 regulations, but the main difference is that ISO 27001 applies to various industries, while HITRUST focuses on high-risk sectors like healthcare. Moreover, HITRUST covers a broader range of data security requirements, including ISO 27001 standard, HIPAA, and NIST.
- How does complying with HITRUST impact healthcare IT projects?
Complying with HITRUST from an early development stage will streamline product development, certification, and implementation, as this regulation unites all sensitive data security standards for medical device development in the USA.
Following HITRUST in your healthcare software development requires establishing security policies that include data encryption, access control, authentication, and data access management. In the long run, it also increases the credibility of your medical solution in the market, increasing sales among healthcare organizations.
Emergency Medical Treatment and Labor Act
The Emergency Medical Treatment and Labor Act (EMTALA) is another federal law regulating healthcare processes. It ensures all patients have access to emergency medical services and are treated correspondingly despite their insurance plans.
Many healthcare units still don't comply with EMTALA, mostly due to the lack of cost and knowledge of the law. However, most of them keep developing strategies for adopting EMLATA.
- How does EMTALA compliance influence medical device development?
EMLATA compliance applies to medical software and hardware devices used for the delivery of emergency medical care. Adhering to its rules at the early development stage is essential to build a compliant solution.
Medical systems designed for emergency departments should provide quick access to patient medical records and triage protocols and be easy to use for physicians. Compliance with EMLATA helps increase the quality and efficiency of emergency treatment and improve patient safety.
CMS Interoperability and Patient Access
This law aims to simplify patient access to their medical records and improve secure data exchanges between different healthcare IT systems. It provides standardized APIs to be integrated into medical systems to ensure smooth data interoperability, high-security protection, and easy access for authorized parties.
CMS Interoperability and Patient Access (Final Rule) helps healthcare providers improve coordination and communication between healthcare units, including hospitals, pharmacies, labs, etc. This approach ensures improved patient care and treatment outcomes.
- How does CMS Interoperability and Patient Access affect healthcare software development?
Medical software compliance with CMS Interoperability and Patient Access regulation is obligatory on a legal level. Each IT system that collects or stores patient health information must adhere to interoperability rules.
To develop a solution that is compliant with this standard, it is necessary to create a software architecture that includes the implementation of standardized APIs, intuitive interfaces for all users, and data security measures.

Importance of compliance with standards and regulations in healthcare product development
As of today, over 14 million individuals suffered from healthcare data breaches in US healthcare facilities in 2024 caused by the lack of security measures and medical device compliance issues.
Developing non-compliant medical solutions exposes healthcare units and patients to critical security vulnerabilities and sensitive information losses. Still, it can also ruin your business reputation and lead to fines and legal issues.
Unlike the EU, a regulation covering AI is currently unavailable in the US. The US government is taking steps towards establishing regulation, so we keep monitoring the status. Find more information on the EU AI Act at the link.
How do you ensure your medical device is compliant?
There are two possible ways: fill in the needs with internal expertise or outsource medical device development to a tech partner.
Choosing the second option, make sure your healthcare software development company is proficient in applicable regulations and complies with industry standards.
This way, you ensure your medical solution gets:
- Architecture design that adheres to the regulatory requirements.
- Robust quality assurance.
- Regular code review and improvements.
- Change management and documentation.
- Compliance assessment throughout the development process.
At Lemberg Solutions, we have over 15 years of experience in high-risk industries like healthcare, and we know how important it is to explore and comply with industry standards and regulations in medical solution development. Regulations compliance ensures medical devices are safe for patients, applicable to the market you’re targeting, and scalable for further improvements.
Feel free to contact our industry experts to discuss your healthcare project and plan the development.